Answer:
Solution A that will form a precipitate with Ksp = 2.3 x 10−4
Step-by-step explanation:
Li₃PO₄ ⇄ 3 Li⁺(aq) + PO₄³⁻(aq)
3S S
Where S = Solubility(mole/lit) and Ksp = Solubility product
⇒ Ksp = (3S)³ x (S)
⇒ 27S⁴ = 2.3x10−4
⇒ S = 0.05 mol/lit
Concentration of Li₃PO₄ precipitate = 0.05
Solution A
0.500 lit of a 0.3 molar LiNO₃ contains 0.5 x 0.3 = 0.15 mole
0.4 lit of a 0.2 molar Na₃PO₄ contains = 3 x 0.4 x 0.2 = 0.24 mole
3 LiNO₃ + Na₃PO₄ → 3 NaNO₃ + Li₃PO₄
(Mole/Stoichiometry)
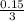
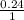
= 0.05 = 0.24
Since from (Mole/Stoichiometry) ratio we can conclude that LiNO₃ is limiting reagent.
So concentration of Li₃PO₄ is equal to 0.05.