Answer:
the partial pressure of the
 at equilibrium is 1.25 atm
at equilibrium is 1.25 atm
Step-by-step explanation:
Initial pressure of the
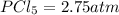
The value of the equilibrium constant =
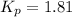
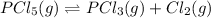
Initially
2.75 atm 0 0
At equilibrium
(2.74- x) x x
The expression for
 for the given reaction
for the given reaction
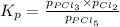
we get:
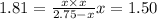
Pressure of the
 at equilibrium :
at equilibrium :
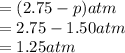
Hence, the partial pressure of the
 at equilibrium is 1.25 atm
at equilibrium is 1.25 atm