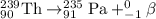Answer:
For A: The equation is
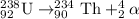
For B: The equation is
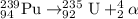
For C: The equation is
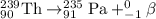
Step-by-step explanation:
Alpha decay process is the process in which nucleus of an atom disintegrates into two particles. The first one which is the alpha particle consists of two protons and two neutrons. This is also known as helium nucleus. The second particle is the daughter nuclei which is the original nucleus minus the alpha particle released.
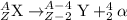
Beta decay process is defined as the process the neutrons get converted into an electron and a proton. The released electron is known as the beta particle. In this process, the atomic number of the daughter nuclei gets increased by a factor of 1 but the mass number remains the same.
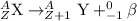
For A: Uranium-238 emits an alpha particle
The nuclear equation for this process follows:
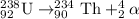
For B: Plutonium-239 emits an alpha particle
The nuclear equation for this process follows:
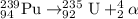
For C: Thorium-239 emits a beta particle
The nuclear equation for this process follows: