Answer:
molar ratio 1:2 (1 mol HCl=2 mol of base)
Step-by-step explanation:
We have the same concentration for the acid
 and the base and we have half of the volume therefore we have to keep in mind that we have a 1:2 molar ratio.
and the base and we have half of the volume therefore we have to keep in mind that we have a 1:2 molar ratio.
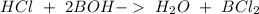
If we have a 1:2 molar ratio between the acid and the base we will need half of the volume of the base.