Answer: 1. neither the forward nor the reverse reactions have stopped.
Step-by-step explanation:
The reactions which do not go to completion and in which the reactant forms product and the products goes back to the reactants simultaneously are known as equilibrium reactions.
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.
For the given reaction:
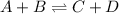
![K=([C]* [D])/([A]* [B)}](https://img.qammunity.org/2021/formulas/chemistry/high-school/hic08trkez3x4obm1dip6fbpdpbwxhf3n7.png)
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
A chemical equilibrium is dynamic in nature i.e. forward and backward reactions continue for indefinite time and never stops.