Answer:
pH = 1.27
Step-by-step explanation:
The reaction is:
HCl(aq) ⇄ H⁺(aq) + Cl⁻(aq)
We have:
m HCl = 1.46 g
V = 750 ml = 0.750 L
M HCl = 36.458 g/mol
We know that 1 mol is equal to:
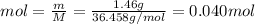
Now, we can find the concentration of HCl:
![[HCl] = (mol)/(V) = (0.040 mol)/(0.750 L) = 0.053 mol/L](https://img.qammunity.org/2021/formulas/chemistry/high-school/s9rqt6ksyesz81mtuuxqaf2f1twl6hjnss.png)
Finally, the pH of the solution is:
![pH = -log [H^(+)] = -log [0.053] = 1.27](https://img.qammunity.org/2021/formulas/chemistry/high-school/ntnl32j9rl3ki1m4t3wkms4shq29f1ayll.png)
Therefore, the ph of a solution that was prepared with 1.46 g of hcl to produce 750 ml of aqueous solution is 1.27.
I hope it helps you!