Answer : The value of work done for the system is 1.821 kJ
Explanation :
First we have to calculate the moles of
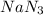

Molar mass of
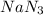 = 65.01 g/mole
= 65.01 g/mole
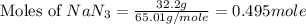
Now we have to calculate the moles of nitrogen gas.
The balanced chemical reaction is,
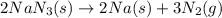
From the balanced reaction we conclude that
As, 2 mole of
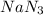 react to give 3 mole of
react to give 3 mole of

So, 0.495 moles of
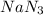 react to give
react to give
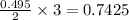 moles of
moles of

Now we have to calculate the volume of nitrogen gas.
Using ideal gas equation:

where,
P = Pressure of
 gas = 1.00 atm
gas = 1.00 atm
V = Volume of
 gas = ?
gas = ?
n = number of moles
 = 0.7425 mole
= 0.7425 mole
R = Gas constant =
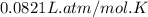
T = Temperature of
 gas =
gas =
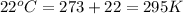
Putting values in above equation, we get:
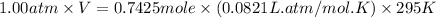
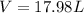
As initially no nitrogen was present. So,
Volume expanded = Volume of nitrogen evolved
Thus,
Expansion work = Pressure × Volume
Expansion work = 1.00 atm × 17.98 L
Expansion work = 17.98 L.atm
Conversion used : (1 L.atm = 101.3 J)
Expansion work = 17.98 × 101.3 = 1821.374 J = 1.821 kJ
Therefore, the value of work done for the system is, 1.821 kJ