Answer:
a) Limiting: sulfur. Excess: aluminium.
b) 1.56g Al₂S₃.
c) 0.72g Al
Step-by-step explanation:
Hello,
In this case, the initial mass of both aluminium and sulfur are missing, therefore, one could assume they are 1.00 g for each one. Thus, by considering the undergoing chemical reaction turns out:
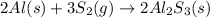
a) Thus, considering the assumed mass (which could be changed based on the one you are given), the limiting reagent is identified as shown below:
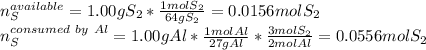
Thereby, since there 1.00g of aluminium will consume 0.0554 mol of sulfur but there are just 0.0156 mol available, the limiting reagent is sulfur and the excess reagent is aluminium.
b) By stoichiometry, the produced grams of aluminium sulfide are:
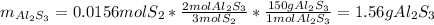
c) The leftover is computed as follows:
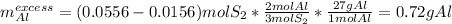
NOTE: Remember I assumed the quantities, they could change based on those you are given, so the results might be different, but the procedure is quite the same.
Best regards.