Answer:
The correct answer is :
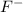
Step-by-step explanation:
According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
Acid after donating protons changes into conjugate base and base after accepting protons changes into conjugate acid.
So, when HF in water reacts, it form fluoride ions and hydronium ions. The recation is given as:
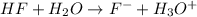
Here, HF is acting as a Bronsted Lowry acid by donating proton and forms it conjugate base
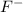 , Where as water is acting as a Bronsted Lowry base by accepting a proton and forms its conjugate acid
, Where as water is acting as a Bronsted Lowry base by accepting a proton and forms its conjugate acid
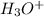 .
.