Answer:
rate = 0.00383 M/s
Step-by-step explanation:
The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction
2Al(s) + 3 CuCl2(aq) → 2AlCl3(aq) + 3 Cu(s)
The equation for reaction rate in this chemical reaction (for CuCl₂) is the following one (The value 1/3 comes from the coefficient in the chemical equation for the reaction):
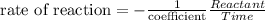
Here, the negative sign is used to indicate the decreasing concentration of the reactant.
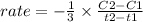
rate = - 1/3 [0.655 - 1.0 / 30]
rate = -1/3[-0.0115]
rate = 3.83 x 10-3 M/s
rate = 0.00383 M/s