Answer:
The balanced chemical equation is given as:
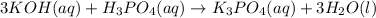
Step-by-step explanation:
When potassium hydroxide reacts with phosphoric acid it gives potassium phosphate and water as a products.
The balanced chemical equation is given as:
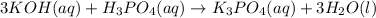
According to reaction, 3 moles of potassium hydroxide recats with 1 mole of phosphoric acid to give 1 mole of potassium phosphate and 3 moles of water.