Answer:
The value of dissociation constant of the monoprotic acid is
 .
.
Step-by-step explanation:
The pH of the solution = 2.46
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![2.46=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/3s1qz0i7a7q29z0whzcpds5bu60s7pr4pm.png)
![[H^+]=0.003467 M](https://img.qammunity.org/2021/formulas/chemistry/college/y72h35uqynvgzyvrk2okszchjcfjj4pumv.png)
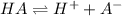
Initially
0.0144 0 0
At equilibrium
(0.0144-x) x x
The expression if an dissociation constant is given by :
![K_a=([A^-][H^+])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/high-school/n5xvssrqsuz68kas50gyuv3j3mnhrgjfp7.png)
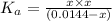
![x=[H^+]=0.003467 M](https://img.qammunity.org/2021/formulas/chemistry/college/j3x7pdpn6kc41h27rid2i67bxqfonzjn3r.png)
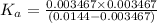
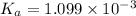
The value of dissociation constant of the monoprotic acid is
 .
.