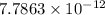Answer : The value of
 is
is
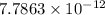
Explanation :
The solubility equilibrium reaction will be:
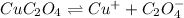
The expression for solubility constant for this reaction will be,
![K_(sp)=[Cu^(+)][C_2O_4^(-)]](https://img.qammunity.org/2021/formulas/chemistry/college/8u1mj4fawonwbo2g0a2cn8ss1axr1oa2x6.png)
Concentration of copper ion = Concentration of oxalate ion = 0.0000027904 M
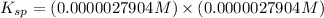
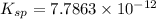
Therefore, the value of
 is
is