Answer:
The work done is 3.4 × 10⁵ J.
Step-by-step explanation:
Given:
Pressure of the gas produced (P) = 179 kPa
Volume of the gas produced (ΔV) = 1.90 m³
We need to find the work done in joules. For that, we don't need any conversion as the units are already in SI units which will give the result in Joules only.
Now, let us verify our results by using conversion factors and without using them.
Using conversion factors:
1 m³ = 1000 L
So, 1.90 m³ = 1.90 m³ × 1000
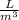 = 1900 L
= 1900 L
Also, 1 atm = 101.325 kPa
So, 179 kPa = 179 kPa ×
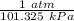 = 1.767 atm
= 1.767 atm
Now, work done in a constant pressure process is given as:
Work = Pressure × Volume change
Work = P × ΔV
Work = 1.767 atm × 1900 L
Work = 3.36 × 10³ atm-L
Now, again using the energy conversion for work.
1 atm-L = 101.325 J
So, 3.36 × 10³ atm-L = 3.36 × 10³ atm-L ×
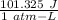 = 3.4 × 10⁵ J
= 3.4 × 10⁵ J
Therefore, the work done is 3.4 × 10⁵ J.
Now, let us verify the above result without any conversion.
Work = P × ΔV = 179 × 1000 × 1.90 = 3.4 × 10⁵ J.
Therefore, the work done is same by both ways.
Hence the work done is 3.4 × 10⁵ J.