Answer:
Succinic Acid
Step-by-step explanation:
We have to start using the info given by the IR spectrum. In this case, we have a broad signal in 3600. This indicates the presence of OH in the structure. Therefore we can have an alcohol or an acid in the structure.
Now, the NMR info tells us that we only have 2 signals, this indicates that we have a very symmetric structure. Also, we have a signal in 12 ppm therefore we can affirm that we have an O-H bond with high polarity (a downfield signal) this behavior is given in the acid groups.
The structure that fulfill these requirements it the succinic acid. In which we only have 2 signals in the 1H- NMR. We have an acid group and we have a formula of
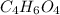 .
.
For further explanations see the attached images.