Answer:
a) molar composition of this gas on both a wet and a dry basis are
5.76 moles and 5.20 moles respectively.
Ratio of moles of water to the moles of dry gas =0.108 moles
b) Total air required = 68.51 kmoles/h
So, if combustion is 75% complete; then it is termed as incomplete combustion which require the same amount the same amount of air but varying product will be produced.
Step-by-step explanation:
Let assume we have 100 g of mixture of gas:
Given that :
Mass of methane =75 g
Mass of ethane = 10 g
Mass of ethylene = 5 g
∴ Mass of the balanced water: 100 g - (75 g + 10 g + 5 g)
Their molar composition can be calculated as follows:
Molar mass of methane
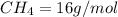
Molar mass of ethane
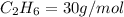
Molar mass of ethylene
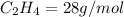
Molar mass of water
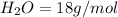
number of moles =

Their molar composition can be calculated as follows:
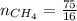
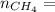 4.69 moles
4.69 moles
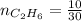
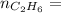 0.33 moles
0.33 moles
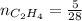
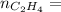 0.18 moles
0.18 moles
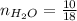
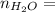 0.56 moles
0.56 moles
Total moles of gases for wet basis = (4.69 + 0.33 + 0.18 + 0.56) moles
= 5.76 moles
Total moles of gas for dry basis = (5.76 - 0.56)moles
= 5.20 moles
Ratio of moles of water to the moles of dry gas =
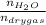
=
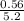
= 0.108 moles
b) If 100 kg/h of this fuel is burned with 30% excess air(combustion); then we have the following equations:

4.69 2× 4.69
moles moles

0.33 3.5 × 0.33
moles moles

0.18 3× 0.18
moles moles
Mass flow rate = 100 kg/h
Their Molar Flow rate is as follows;

Total moles of
 required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles
required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles
= 11.075 k moles.
In 1 mole air = 0.21 moles

Thus, moles of air required =
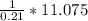
= 52.7 k mole
30% excess air = 0.3 × 52.7 k moles
= 15.81 k moles
Total air required = (52.7 + 15.81 ) k moles/h
= 68.51 k moles/h
So, if combustion is 75% complete; then it is termed as incomplete combustion which require the same amount the same amount of air but varying product will be produced.