Answer:
0.5 M is the required molarity of a barium hydroxide solution to prepare a 1.0 M of hydroxide solution.
Step-by-step explanation:
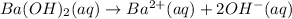
Let the molarity of barium hydroxide be x.
According to reaction , 2 M of hydroxide ions are obtained from 1 M of barium hydroxide
Then 1.0 M of hydroxide ion swill be obtained from:
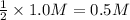 of barium hydroxide
of barium hydroxide
0.5 M is the required molarity of a barium hydroxide solution to prepare a 1.0 M of hydroxide solution.