Answer:
1.457*10^-8 grams
Step-by-step explanation:
First we want to find the molar concentration of MgF2. We can do this by dividing 0.016 (the solubility in grams of MgCl2 in a litre of water) by its molar mass (approx. 62.3 grams). Thus, the molar solubility of MgF2 is 2.57*10^-4 M.
Next, we must calculate the Ksp of MgF2. The equilibrium expression is:
MgF2⇄Mg+2F
Thus
![Ksp=[Mg^+][F^-]^2](https://img.qammunity.org/2023/formulas/chemistry/college/w4bzatgeig7r7y9c4ad9kohzcgz78wfhvt.png)
This means that, in equilibrium, there are 2.57*10^-4 M of
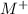 and 5.136*10^-4 M of
and 5.136*10^-4 M of
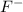
Plugging in the above information, our Ksp for MgF2 is approximately 6.78*10^-11
Next we will need to use the RICE table. Since there is already 0.29M of NaF dissolved, there is initially 0.29M of
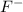 .
.
R: MgF2 ⇄
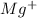 +2
+2
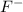
I: N/A 0 0.29M
C: N/A +x +x
--------------------------------------------
E: N/A x 0.29+x
To make calculations easier, we will assume that 0.29+x≈0.29
This means that Ksp=0.29x=6.78*10^-11
Therefore, x≈2.338*10^-10M
Multiply that by 62.3 and we get around 1.457*10^-8 grams.