Answer:
The volume of carbon dioxide at 20.0°C and 0.941 atm produced was 6390.89 Liters.
More volume of carbon dioxide gas was released on combustion of 4.00 kg of propane in comparison to the volume of carbon dioxide released on combustion of 4.00 kg of methane.
Step-by-step explanation:
Methane
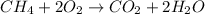
Mass of methane = 4.00 kg = 4000 g (1 kg = 1000 g)
Moles of methane =
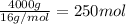
According to reaction, 1 mole of methane gives 1 mole of carbon dioxide gas,then 250 moles of methane will give :
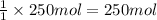 of carbon dioxide gas
of carbon dioxide gas
Moles of carbon dioxide gas = n = 250 mol
Pressure of carbon dioxide gas = P = 0.941 atm
Temperature of carbon dioxide gas = T = 20.0°C = 20.0+273 K = 293 K
Volume of carbon dioxide gas = V
 (Ideal gas equation)
(Ideal gas equation)
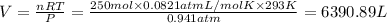
The volume of carbon dioxide at 20.0°C and 0.941 atm produced was 6390.89 Liters.
Propane
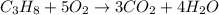
Mass of propane = 4.00 kg = 4000 g (1 kg = 1000 g)
Moles of propane =
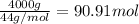
According to reaction, 1 mole of propane gives 3 mole of carbon dioxide gas,then 90.91 moles of propane will give :
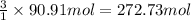 of carbon dioxide gas
of carbon dioxide gas
Moles of carbon dioxide gas = n = 272.73 mol
Pressure of carbon dioxide gas = P = 0.941 atm
Temperature of carbon dioxide gas = T = 20.0°C = 20.0+273 K = 293 K
Volume of carbon dioxide gas = V
 (Ideal gas equation)
(Ideal gas equation)
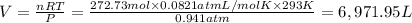
The volume of carbon dioxide at 20.0°C and 0.941 atm produced was 6,971.95 Liters.
Volume of carbon dioxide given by combustion of 4.00 kg of methane = 6390.89 Liters.
Volume of carbon dioxide given by combustion of 4.00 kg of propane = 6,971.95 Liters.
6390.89 Liters < 6,971.95 Liters
More volume of carbon dioxide gas was released on combustion of 4.00 kg of propane in comparison to the volume of carbon dioxide released on combustion of 4.00 kg of methane.