Answer:
The molar solubility of the metal thiocyanate is
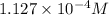 .
.
Step-by-step explanation:
Concentration of potassium thiocyanate = 0.421 M
Concentration of thiocyanate ion =
![[SCN^-]= 0.421 M](https://img.qammunity.org/2021/formulas/chemistry/college/jk4u2e4xql7b1zbyiutayyqffoefs0kn7u.png)
Concentration of metal ion =
![[M^(2+)]= ?](https://img.qammunity.org/2021/formulas/chemistry/college/nye6oz3eurptapon6cdixljpwe1s63t8af.png)
The solubility product of metal thiocyanate =
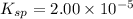
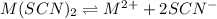
S 2S
At equilbrium
S (2S+0.421)
The expression of solubility product is given by :
![K_(sp)=[M^(2+)]* [SCN^-]^2](https://img.qammunity.org/2021/formulas/chemistry/college/j8fd6574gpx4do4qx9xrzuoqhz72abz6um.png)
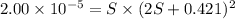
Solving for S:
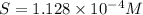
![[M^(2+)]=(2.00* 10^(-5))/((0.421 M)^2)=1.127* 10^(-4) M](https://img.qammunity.org/2021/formulas/chemistry/college/ol7w6d12qsix2lbyzedlb2ap6i5ua3lss8.png)
The molar solubility of the metal thiocyanate is
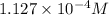 .
.