Answer:
The entropy change for the vaporization of 18 grams of a hydrocarbon at its boiling point is 12.87 J/K.
Step-by-step explanation:
Mass of hydrocarbon = 18 g
Molar mass of hydrocarbon = 114 g/mol
Moles of hydrocarbons =
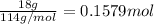
The enthalpy of vaporization of hydrocarbon =
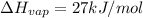
Heat required to heat 0.1579 moles of hydrocarbon = Q
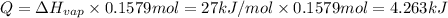
Q = 4.263 kJ = 4,263 J ( 1 kJ = 1000 J)
Boiling point of hydrocarbon = T = 58.2°C = 58.2 + 273 K = 331.2 K
Entropy change for the vaporization of 18 g of a hydrocarbon:
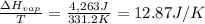
The entropy change for the vaporization of 18 grams of a hydrocarbon at its boiling point is 12.87 J/K.