Answer:
Option (E) is correct
Step-by-step explanation:
Solubility equilibrium of
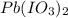 is given as follows-
is given as follows-
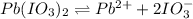
Hence, if solubility of
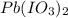 is S (M) then-
is S (M) then-
![[Pb^(2+)]=S(M)](https://img.qammunity.org/2021/formulas/chemistry/high-school/q888vjiepi80ctxl65et9qoackauhnhjh8.png) and
and
![[IO_(3)^(-)]=2S(M)](https://img.qammunity.org/2021/formulas/chemistry/high-school/g2sug33lv7dwoetd0objr5nk9uw8w54n4p.png)
Where species under third bracket represent equilibrium concentrations
So, solubility product of
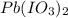 ,
,
![K_(sp)=[Pb^(2+)][IO_(3)^(-)]^(2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/7r6yjq6d76i54h85oaqldusba47b6b6556.png)
Here,
![[Pb^(2+)]=S(M)=5.0* 10^(-5)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/doosyo9kqfsw51uy6jp24ybif8syf1zzfx.png)
So,
![[IO_(3)^(-)]=2S(M)=(2* 5.0* 10^(-5))M=1.0* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/wtkrq16jrw4mj5jh2z6dn95w8wd7mrh6e6.png)
So,
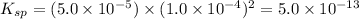
Hence option (E) is correct