Answer:
0.3428 g/mL is the concentration that student will write in her notebook.
Step-by-step explanation:
Mass of the empty graduated cylinder, w = 2.2 g
Mass of sodium thiosulfate and graduated cylinder ,w'= 19.440 g
Mass of sodium thiosulfate : W
= w' - w = 19.440 g - 2.2 g = 17.24 g
Volume of the solution after adding water to the graduate cylinder with sodium thiosulfate = V
V = 50.29 mL
According to question :
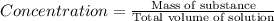
Concentration of the solution :
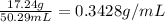
0.3428 g/mL is the concentration that student will write in her notebook.