Answer: The net chemical equation for the formation of manganese from manganese (II) carbonate, oxygen and aluminum is written above.
Step-by-step explanation:
The given chemical equation follows:
Equation 1:
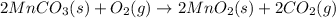 ( × 3)
( × 3)
Equation 2:
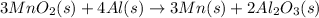 ( × 2)
( × 2)
As, the net chemical equation does not include manganese (IV) oxide. So, to cancel out from the net equation, we need to multiply equation 1 by (3) and equation 2 by (2)
Now, the net chemical equation becomes:
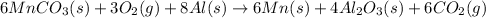
Hence, the net chemical equation for the formation of manganese from manganese (II) carbonate, oxygen and aluminum is written above.