Answer :
(1) The number of cups needed are, 0.45 cups.
(2) The number of cups needed are, 5 cups.
Explanation :
Part 1:
First we have to calculate the moles of NaCl.
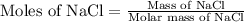
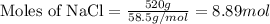
Now we have to calculate the number of cups needed.
As, the number of cups needed for 8.89 mole of NaCl = 1 cups
So, the number of cups needed for 4 mole of NaCl =
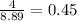 cups
cups
Thus, the number of cups needed are, 0.45 cups.
Part 2:
Given:
Mass of ice cubes = 1 kg = 1000 g
Now we have to calculate the number of cups needed.
As, the number of cups needed for 220 g of ice = 1 cups
So, the number of cups needed for 1000 g of ice =
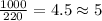 cups
cups
Thus, the number of cups needed are, 5 cups.