Answer:
0.635 grams
Step-by-step explanation:
Equation for the reaction

mass of Cu = 5.00 g
molar mass = 63.5 g/mol
number of moles =
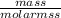
number of moles of Cu =
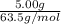
number of moles of Cu = 0.0787 moles
To determine the moles of Ag formed; we have:
0.00588 moles of AgNO₃ ×
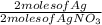
= 0.00588 moles of Ag are produced
Molar mass of Ag = 108 g/mol
Then mass of Ag that will be produced = number of moles of Ag × molar mass of Ag
= 0.00588 moles × 108 g/mol
= 0.635 grams of Ag are produced.