Answer : The heat your body transfer must be, 25.1 kJ
Explanation :
Formula used :
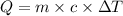
or,
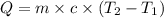
where,
Q = heat = ?
m = mass of water = 500.0 g
c = specific heat of water =
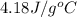
 = initial temperature =
= initial temperature =
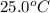
 = final temperature =
= final temperature =
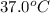
Now put all the given value in the above formula, we get:
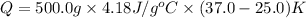
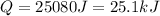
Therefore, the heat your body transfer must be, 25.1 kJ