0.0018 grams is the mass of silver can 2 kJ of energy raise 15°C.
Step-by-step explanation:
Data given:
heat supplied (q) = 2KJ or 2000 J
cp of silver= 0.24 j/gram °C (specific heat capacity is the amount of heat required to raise the temp of substance by 1 degree)
temperature = 15 degree centigrade
mass=?
using the equation for the heat capacity,
q= mcpT
m =
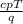
Putting the values in the equation:
=
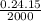
= 0.0018 grams is the mass of silver in which 2 kJ of energy raise temperature by 15°C.