Answer:
34.15% is the mass percentage of calcium in the limestone.
Step-by-step explanation:
Mass of precipitate that is calcium oxalate = 140.2 mg = 0.1402 g
1 mg = 0.001 g
Moles of calcium oxalate =
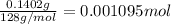
1 mole of calcium oxalate have 1 mole of calcium atom.
Then 0.001095 moles of calcium oxalate will have 0.001095 moles of calcium atom.
Mass of 0.001095 moles of calcium :
0.001095 mol × 40 g/mol = 0.04381 g
Mass of sample of limestone = 128.3 mg = 0.1283 g
Percentage of calcium in limestone:
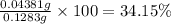
34.15% is the mass percentage of calcium in the limestone.