Answer: It is a Lewis acid/base reaction, and
 is the Lewis acid.
is the Lewis acid.
Step-by-step explanation:
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
 can readily accept electrons and thus act as a lewis acid which is short of electrons.
can readily accept electrons and thus act as a lewis acid which is short of electrons.
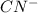 can readily lose electrons and thus act as a lewis base which has excess of electrons.
can readily lose electrons and thus act as a lewis base which has excess of electrons.
It is a Lewis acid/base reaction, and
 is the Lewis acid.
is the Lewis acid.