Answer:
92.3g
Step-by-step explanation:
Given parameters:
Mass of water = 540g
Percentage by mass of solution = 15%
Unknown:
Mass of sucrose = ?
Solution:
A solution is a mixture made up of solutes dissolved in a solvent. The solvent is the liquid and the solute is usually the solid.
Solution = solute + solvent
To find the weight percent of solute in solution ;
=
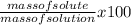
Mass of solution = mass of sucroce + water
= S + 540
where S is the mass of sucrose;
therefore;
15 =
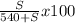
solving for S;
S = 92.3g