Answer:
Water
Step-by-step explanation:
To solve this problem, we need to solve for the number of molecules of these compounds;
given:
Mass of water = 1.25g
Mass of C₂H₅OH = 0.89g
We first solve for the number of moles of these compounds;
Number of moles =

Molar mass of H₂O = (2x1) + 16 = 18g/mole
Molar mass of C₂H₅OH = (12 x 2) + (1 x 5) + 16 + 1 = 46g/mole
Number of moles of H₂O =
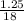 = 0.07moles
= 0.07moles
Number of moles of C₂H₅OH =
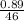 = 0.02moles
= 0.02moles
We see that number of moles of water is more than that of ethanol. Therefore it has more molecules because;
1 mole of substance = 6.02 x 10²³
0.02moles of ethanol = 1.2 x 10²²molecules of ethanol
0.07moles of water = 4.2 x 10²² molecules of water