Answer:
True
Step-by-step explanation:
The number density of an ideal gas at stp is called the loschmidt number, being named after the Austrian physicist Johann Josef Loschmidt who first estimated this quantity in 1865
n =
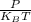
where
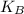 is the Boltzman constant
is the Boltzman constant
T is the temperature
P is the pressure
Loschmidt Number is the number of molecules of gas present in one cubic centimetre of it at STP conditions.Loschmidt constant is also used to define the amagat, which is a practical unit of number density for gases and other substances:
1 amagat = n = 2.6867811×1025 m−3,