Answer:
P₂ = 5.55 atm
Step-by-step explanation:
Mathematically, Boyle's law can be stated as:
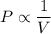
Pressure is inversely proportional to the volume
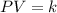 Pressure multiplied by volume equals some constant
Pressure multiplied by volume equals some constant
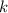
where P is the pressure of the gas, V is the volume of the gas, and k is a constant.
The equation states that the product of pressure and volume is a constant for a given mass of confined gas and this holds as long as the temperature is constant. For comparing the same substance under two different sets of conditions, the law can be usefully expressed as:
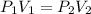
The final pressure of a gas is calculated using Bolyes law formula
that is P1V1 =P2V2
Because the temperature and number of moles remained constant, we can use the formula
P₁V₁=P₂V₂
3.00 L * 1.85 atm = P₂ * 1.00 L
P₂ = 5.55 atm