Answer:
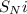 is the major step in forming acid chloride from carboxylic acid and thionyl chloride
is the major step in forming acid chloride from carboxylic acid and thionyl chloride
Step-by-step explanation:
- In the first step, -OH group in carboxylic acid gives nucleophilic substitution reaction at S center in thionyl chloride and substitutes -Cl atom
- In the second step, deprotonation takes place by chloride ion.
- In the third step, an intramolecular nucleophilic substitution reaction (
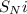 ) takes place where bond electrons rearranges to produce
) takes place where bond electrons rearranges to produce
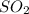 , HCl and thionyl chloride.
, HCl and thionyl chloride. - This rearrangement is highly favorable due to formation of gaseous species
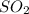
- Reaction mechanism has been shown below.