2075g of XeF₆ is present in 8.46 moles of XeF₆
Step-by-step explanation:
Given:
XeF₆ is xenon hexafluoride
number of moles of XeF₆ is 8.46
Mass of XeF₆ present, m = ?
We know,
Molecular weight of XeF₆, M is 245.28 g/mol
and
number of moles, n = mass of XeF₆ present / molecular weight of XeF6
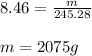
Therefore, 2075g of XeF₆ is present in 8.46 moles of XeF₆