The question is incomplete, here is the complete question:
A chemist dissolves of 716. mg pure potassium hydroxide in enough water to make up 130. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25°C.) Round your answer to 2 significant decimal places.
Answer: The pH of the solution is 13.14
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
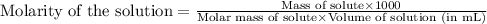
Given mass of NaOH = 716 mg = 0.716 g (Conversion factor: 1 g = 1000 mg)
Molar mass of NaOH = 40 g/mol
Volume of solution = 130 mL
Putting values in above equation, we get:
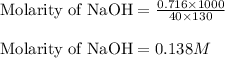
1 mole of NaOH produces 1 mole of
 ions and 1 mole of
ions and 1 mole of
 ions
ions
To calculate the pOH of the solution, we use the equation:
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
We are given:
![[OH^-]=0.138M](https://img.qammunity.org/2021/formulas/chemistry/college/eny8bewnuixndqetoid6yux3dye5y47jer.png)
Putting values in above equation, we get:
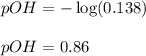
To calculate pH of the solution, we use the equation:
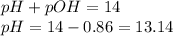
Hence, the pH of the solution is 13.14