2. 15.65 grams of KCl is formed if 25.0 g of Potassium Chlorate, KClO3, decompose.
3. 2.17 moles of H2 gas produced when 100.0 grams of Na is added to the reaction.
4. 7.5 moles of Hydrogen, H2, are needed to react with 2.5 moles of Nitrogen, N2.
Step-by-step explanation:
2. The balanced equation for the chemical reaction is:
2KClO3 ⇒ 2KCl + 3O2
Data given:
mass of KClO3 = 25 grams, atomic mass of KClO3 = 122.55 grams/mole
KCl produced =? atomic mass of KCl = 74.55
number of moles =
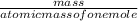
=
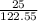
number of moles = 0.20 moles of KCO3
2 moles of KClO3 decomposes to give 2KCl
0.21 moles of KClO3 decomposes to give x moles of KCl
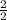 =
=
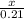
x = 0.21 moles of KCl
mass of KCl = 0.21 x 74.55
= 15.65 grams of KCl is formed.
3. data given:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
mass of Na added = 100 grams, atomic mass of Na = 22.98 grams/mole
moles of H2 =?
Number of moles of Na =
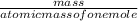
=
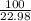
number of moles of Na = 4.35 moles
2 moles of Na gives 1 mole of H2
4.35 moles of Na will give x moles
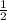 =
=
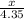
2x = 4.35
x = 2.17 moles of hydroden gas is produced.
4. Data given:
the balanced chemical equation:
N2 + 3H2 → 2NH3
Number of moles of N2 2.5
from the reaction
1 mole of N2 reacts with 3 moles of H2
The molar ratio of the reactant is 1:3
so,
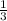 =
=
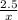
x = 7.5 moles of H2 will react with 2.5 moles of N2.