Hello!
A gas that has a volume of 28 liters, a temperature of 42°C, and an unknown pressure has its volume increased to 49 liters and its temperature decreased to 27°C. If the pressure is measured after the change at 4.0 atm, what was the original pressure of the gas?
We have the following data:
P1 (initial pressure) = ? (in atm)
V1 (initial volume) = 28 L
T1 (initial temperature) = 42ºC (in Kelvin)
TK = TºC + 273.15
TK = 42 + 273.15 → T1 (initial temperature) = 315.15 K
P2 (final pressure) = 4 atm
T2 (final temperature) = 27ºC (in Kelvin)
TK = TºC + 273.15
TK = 27 + 273.15 → T2 (final temperature) = 300.15 K
V2 (final volume) = 49 L
Now, we apply the data of the variables above to the General Equation of Gases, let's see:
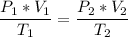
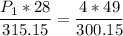
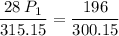
multiply the means by the extremes
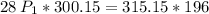
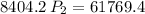
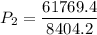
Answer:
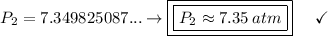
The original pressure of the gas is approximately 7.35 atm
_______________________