0.0017 litres of H2 will be formed if 40.0 grams of sodium react with excess hydrochloric acid.
The balanced equation for the reaction:
2Na +2 HCl ⇒ H2 +2 NaCl
Step-by-step explanation:
the balanced equation for the reaction:
2Na +2 HCl ⇒ H2 +2 NaCl
data given:
Temperature of the reaction = 280 k
pressure of the reaction = 96 kPa
atomic mass of H2 = 2.01 grams/mole
mass of sodium Na = 40 grams
atomic mass of sodium = 22.98 grams/mole
number of moles =
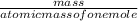
Number of moles =
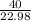
= 1.7 moles of Na will react.
from, the equation 2 moles of Na reacts to form 1 mole of H2
1.7 moles of Na reacts to form x mole of H2
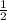 =
=
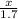
x = 0.85 moles of H2 will be formed.
mass of H2 will be calculated by multiplying number of moles with atomic mass of H2
0.85 x 2.01
= 1.708 grams of H2 will be formed.
To convert grams into litre the value in litres is divided by 1000
as 1kg =1000 grams is equal to 1L = 1000 grams
so 0.0017 litres of H2 will be formed.