287.39 grams of Al2(SO4)3 is formed if 250g H2SO4 completely reacted with aluminum.
Step-by-step explanation:
Balanced chemical equation:
2Al + 3H2SO4->Al2(SO4)3+H2
Data given:
atomic mass of Al2(SO4)3 = 342.14 gram/mole
mass of H2SO4 given = 250 gram
atomic mass of one mole of H2SO4 = 98.079 grams/mole
number of moles =
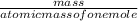
number of moles =
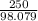
number of moles = 2.54 moles
from the equation it can be seen as
3 moles of H2SO4 will react to give 1 mole Al2(SO4)3
2.54 moles of H2SO4 will react to give x moles of Al2(SO4)3.
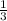 =
=
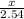
x = 0.84 moles of Al2(SO4)3 is formed.
to calculate mass of the Al2(SO4)3 = Number of moles x atomic weight of one mole
= 0.84 x 342.14
= 287.39 grams of Al2(SO4)3 is formed.