Answer:
Pressure would be approximately 4.66 pounds.
Explanation:
Given:
Volume of gas (V) = 25 cubic cm
Pressure of the gas (P) = 11 pounds
We need to find the pressure when volume is 59 cubic cm.
Solution:
Now Given:
 ∝
∝
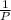
so we can say that;
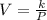
where k is a constant.
When V = 25 cubic cm, P =11 pounds.
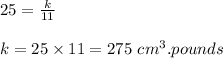
So the equation becomes as.
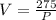
Now we need to find the pressure when Volume is 59 cubic cm.
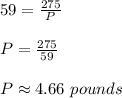
Hence Pressure would be approximately 4.66 pounds.