80 ml or 0.08 L of 1M HCl is needed to make 12 M HCl.
Step-by-step explanation:
We have to use the law of Volumetric Analysis, to find the volume of the 12M solution using the volume and the molarity of the known solution of hydrochloric acid, using the law as,
V₁M₁ = V₂M₂
The above equation can be rewritten to find the volume as,
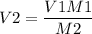
Plugin the given values as,
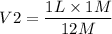 = 0.08 L or 80 ml
= 0.08 L or 80 ml