Answer:
12.8
Step-by-step explanation:
Considering:
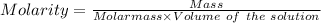
For NaCl:-
Mass = 12.25 g
Molar mass = 58.44 g/mol
Volume of solution = 250.0 mL = 0.25 L
So,
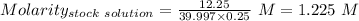
Considering
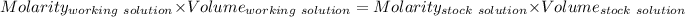
Given that:
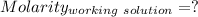
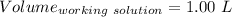
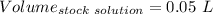
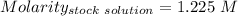
So,
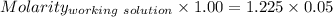
Concentration of NaOH = Concentration of [OH⁻] = 0.06125 M
pOH = - log[OH⁻] = -log(0.06125) = 1.21
pH = 14 - pOH = 14 - 1.21 = 12.8