Answer:
0.51M
Step-by-step explanation:
Given parameters:
Initial volume of NaBr = 340mL
Initial molarity = 1.5M
Final volume = 1000mL
Unknown:
Final molarity = ?
Solution;
This is a dilution problem whereas the concentration of a compound changes from one to another.
In this kind of problem, we must establish that the number of moles still remains the same.
number of moles initially before diluting = number of moles after dilution
Number of moles = Molarity x volume
Let us find the number of moles;
Number of moles = initial volume x initial molarity
Convert mL to dm³;
1000mL = 1dm³
340mL gives
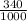 = 0.34dm³
= 0.34dm³
Number of moles = initial volume x initial molarity = 0.34 x 1.5 = 0.51moles
Now to find the new molarity/concentration;
Final molarity =
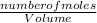 =
=
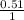 = 0.51M
= 0.51M
We can see a massive drop in molarity this is due to dilution of the initial concentration.