Answer:
Now since mass of reactant is equal to mass of the product after the reaction so we can say that mass conservation is applicable here
Step-by-step explanation:
As we know that zinc reacts with copper sulfate
so the reaction is given as
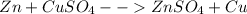
so here we have
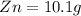
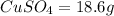
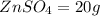
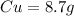
Now total mass of reactant is given as
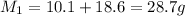
Mass of the product is given as
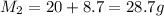
Now since mass of reactant is equal to mass of the product after the reaction so we can say that mass conservation is applicable here