-616 kilo joules of energy is released when 40 grams glucose are combusted.
Step-by-step explanation:
Balance equation of the reaction:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O and ΔH = -2800 kJ
1 mole glucose undergoes combustion to release 2800 kJ of energy
atomic mass of 1 mole of glucose = 180.15 gram/mole
40 grams of glucose will have
number of moles =
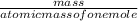
number of moles =
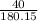
= 0.22 moles of glucose in 40 gram.
1 mole of glucose when undergoes combustion yields -2800 kilo joules of energy
0.22 moles of glucose when undergoes combustion yields
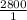 =
=
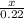
x = -616 kilo joules of energy is released when 40 grams glucose undergoes combustion. (minus sign indicates release of energy)