Answer:
The number of calories needed is 6c.
Step-by-step explanation:
The amount of energy
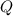 needed to raise the temperature
needed to raise the temperature
 of water of mass
of water of mass
 is
is
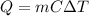
where
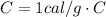 is the specific heat capacity of water.
is the specific heat capacity of water.
Putting in numbers into equation (1), we get:
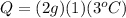
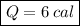
which is the number of calories needed.