40.6 % is the percentage of energy in glucose that is available for the body to use.
Step-by-step explanation:
We know that during cellular respiration one mole of glucose gives 38 molecules of ATP.
Given that 1 mole of ATP gives 30.7 KJ of energy.
So 38 molecules of ATP would give
38 x 30.7 KJ of energy.
= 1166 KJ would be yielded by 38 moles of ATP.
1 mole of glucose contains 2870 KJ of energy.
100 % of energy is released in glucose metabolism and x percent is used. It is given by the equation
2870: 100 = 1166:x
x=
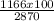
= 40.6 %
40.6% of energy is available for the body to use and rest of the energy is dissipated in the form of heat when 1 mole of glucose undergoes combustion in cellular respiration producing 38 moles of ATP.