Answer:
Mass, M is 440.883g
Step-by-step explanation:
Given the following;
Moles=4.5moles
Molar mass of H3PO4=
Atomic mass of Hydrogen H=(1*3)=3
Atomic mass of Phosphorus P=30.974
Atomic mass of Oxygen O=(16*4)=64
Therefore, Molar mass is;
H3PO4=3+30.974+64=97.974g/mol
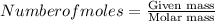
Mass M = Number of moles × Molar mass
M = 4.5 × 97.974
M = 440.883g